Patient Centricity in Clinical Trials: Progress — and Miles to Go

A few years ago, we wrote a blog series for ACRP in which we focused on patient centricity in clinical trial design, recruitment, and execution. Over the next few months, we’ll be taking a second look at those blog posts, sharing updated versions on our own blog. Here’s an update to the first in that series.
If you track trends in web searches of the word “patient” over the last 15 years, an interesting trend emerges: the line goes up, and up, and up. This bears out in trends we’ve observed throughout the years as well: an increase in empowered patients and an increase in patient-focused technologies like patient portals, for example.
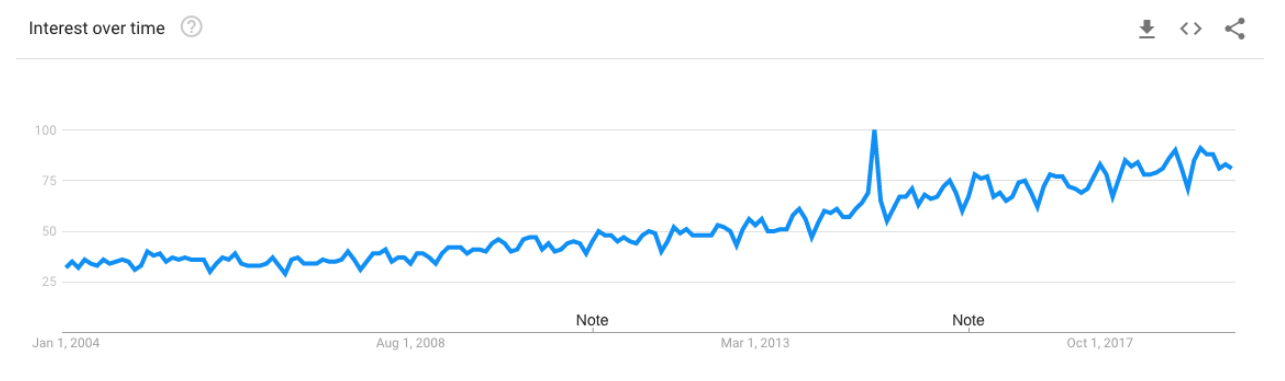
But this trend isn’t paralleled by any great increase in patients taking part in clinical research. The 2015-2016 Global Participation in Clinical Trials Report from the FDA notes that while over those two years,131,749 took part in a “pivotal” clinical trial, that means that less than 2 out of every 1,000 people in the world took part. While “patient” searches are up, there’s lots of work to be done in connecting those patients to medical research.
This means putting the patient at the center of medical research; it means practicing what we preach in terms of patient centricity. Because the fact of the matter is, while there has been some progress and rays of hope, the clinical trial enterprise is still largely the same. It is time to embrace full patient involvement and not just platitudes of patient centricity.
Research remains an expensive, slow-moving endeavor, but the need for new and better treatments remains high. Around half of all American adults live with one or more chronic health conditions — clinical research can help them. In cancer specifically, every year 12.7 million people are diagnosed — clinical research can help them. The fact is that for many diseases, a clinical trial should be considered as a care option — it may be a patient’s best chance, and is typically accompanied by expert care and a full workup.
So how can we ensure that as we wade through the monolith that is industry-sponsored medical research, we are remembering our “why”? How can we keep patients at the center of the work? It needs to be a conscious effort at the time of trial design, and when recruitment begins, and even as data starts to come in from the trial.
First, how can we design trials that are the right fit for patients? There are a lot of resources out there to help make this a reality. The FDA now states that trial design, to begin with, should be driven by patients to make trials less burdensome. Key here is actually asking patients what they need, want, and won’t accept. At Antidote, we recently conducted a survey that revealed the participation motivations and logistical concerns of nearly 4,000 patients — taking findings from patient-driven research like this into account during trial design is an excellent first step.
We know that patient recruitment is also a challenge — 80% of clinical trials are delayed or closed due to difficulty finding patients to take part. To start, traditional patient recruitment is study-centric, not patient-centric. Researchers ask, “does this patient match MY study?” But patients are not looking for a study, they are looking for improved health through treatment options. A fresh approach can shorten recruitment timelines by connecting more patients with appropriate trial opportunities.
And what about the actual execution of a clinical trial? Clinical research professionals play an important role in ensuring the patient experience of each trial is a positive one. In fact, we’ve heard from patients that their interaction with Study Coordinators is what drives them to continue engaging in a study. Retention is key to the success of each trial — so it’s important to remember that patients are critical to the success of a trial, and to treat them accordingly.
At Antidote, we believe in empowering patients to find a trial that’s right for them — and in empowering industry professionals to better engage patients and drive research studies forward.
Topics: For Sponsors

