How to read a clinical trial listing using Antidote’s matching tool

If you’re interested in joining a clinical trial, one of the first places anyone will tell you to look is ClinicalTrials.gov, a database of privately and publicly funded clinical studies conducted around the world. Everyone has access to the key details of clinical trials listed on ClinicalTrials.gov, where you can see eligibility criteria. However, inclusion and exclusion criteria have become increasingly complex, making it more difficult than ever for patients to read clinical trial listings on their own. Listings on ClinicalTrials.gov are also designed for researchers, and most patients can’t understand medical jargon. The academic nature of the listings is further complicated by the way they are presented — they are literally hard to read.
At Antidote, our goal is to connect patients with clinical trial options through a simple search process. Our clinical trial matching engine uses structured data and proprietary algorithms to explore a patient’s eligibility for every trial. The goal of clinical trial matching software is to take complex information included in a trial listing and translate it into a tool as easy to use as the search tools we use every day.
Even with our simplified process and clean and simple design, clinical trial listings can have complex language and unfamiliar terms. If you're having trouble navigating a study page you found, the definitions below can help. We used our clinical trial matching tool to search for open Alzheimer’s disease clinical trials. Here’s some of the terminology you need to know.
Results page
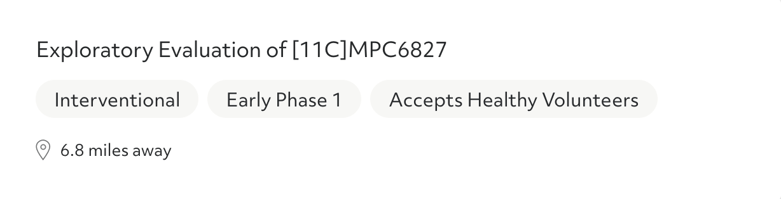

After you answer a few questions, you'll see a list of clinical trial options that you may be eligible for. The results can be filtered by phase and trial type.
Trial phase: In order to reach patients, every potential medical intervention must make its way through a rigorous approval process, passing through clinical trial phases. Each clinical trial phase has a different goal and separate hurdles to clear before a potential new treatment or medical device can move forward.
- Phase I clinical trials test whether drugs are safe to use in humans. These trials are typically small — they enroll approximately 20 to 100 volunteers. Some phase I clinical trials only look for healthy volunteers. Others accept those with the condition the drug aims to target. While the drug may help symptoms, a phase I trial’s goal is to prove safety, but not necessarily how effective the drug or device is. This trial phase can last anywhere from several months to a year.
- Phase II clinical trials test both the safety and effectiveness of a drug or medical device. Trials in this stage can last from several months to a few years, and recruit up to several hundred patients with the condition to take part. Many phase II clinical trials are randomized, which means one group of participants receives the study drug, while the other group receives a placebo or an existing, standard treatment. Other phase II studies are "blinded," which means that neither the researchers nor the participants know who received the study drug.
- Phase III clinical trials are usually the largest — these trials test a potential treatment in hundreds to thousands of people in a randomized, blind study. This testing phase can last several years as the FDA gathers thorough data about the drug's effectiveness and potential side effects.
There are also sub-phases of trials. These trials may be seeking a bit of additional information before submitting results from all of the trials to the FDA.
Trial type: You'll also see here whether a trial is interventional or observational.
- Interventional trials evaluate whether some form of an intervention is safe and effective. This includes a potential drug, medical device, activity, or procedure.
- Observational studies don't test potential treatment options. Instead, researchers observe participants on their current treatment plans and track health outcomes.
Condition area
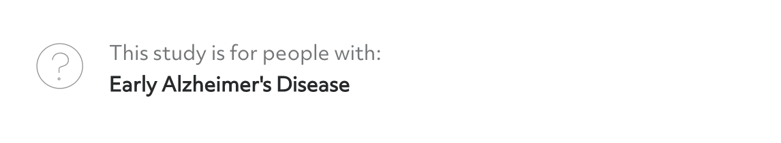
In this section, you'll find the condition area for the study. Sometimes, this information may be specific and mention a subtype of a particular condition. For example, a trial may be for exercise-induced asthma, early Alzheimer’s disease, or a certain kind of multiple sclerosis.
Intervention being studied

This section lets you know what treatment is being researched in the study. An "intervention" can be an experimental medication, device, activity, or procedure.
Purpose of the trial

In this section, you'll see the goal of the trial – basically, why it's being conducted. For example, the purpose of the trial may be to research whether or not an investigational drug is safe and effective for patients. Trials may also research a non-medication option, such as a new method of health care delivery.
You may also see in this section that a trial is observational rather than interventional. In observational trials, patients are not given an intervention. Instead, participants are put into groups and observed. For example, a researcher may divide older adults into groups based on their diet and track health outcomes.
Clinical trial sponsor

This is the pharmaceutical company or research organization running the trial.
Unique study ID

Every trial registered on ClinicalTrials.gov receives a unique study ID.
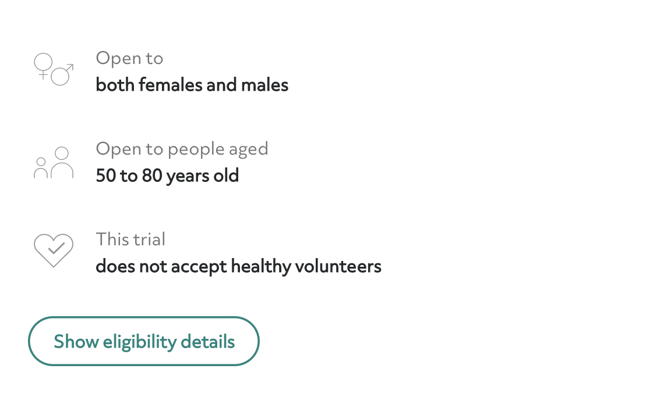
Eligibility requirements
Here, you'll find more information about the inclusion and exclusion criteria for the trial. Clicking “show eligibility details” reveals the entire list of inclusion and exclusion criteria. If you don't qualify for this trial, try looking back at your search results. Every trial has different criteria, so if you don't qualify for one, other options might work for you.

How to learn more about a trial
After you've learned the basics about a trial, the next step is to contact one of the available trial sites. Before you join, you’ll be able to learn more about the study and the screening process. If you don’t know where to start, we’ve created a handy list of 15 questions to ask the study team.
We encourage you to discuss trials you find in our match tool with your doctor. Be sure to talk to them about how the intervention may impact your current treatment plan.
Here's an example of a list of site locations:
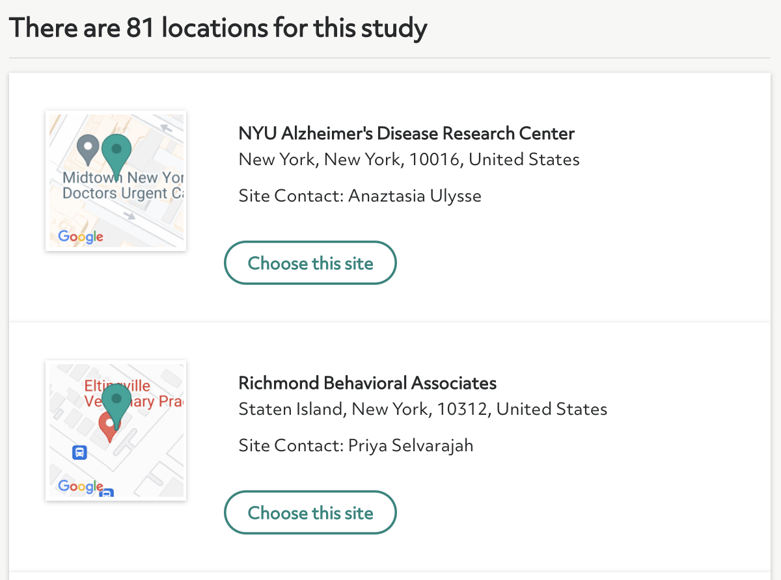
Contact a research site by clicking “choose the site.” You'll see an option to email yourself the contact information for the site. Reach out to the study team at your own convenience using the contact information listed.
This is the screen you'll see after choosing a site location:
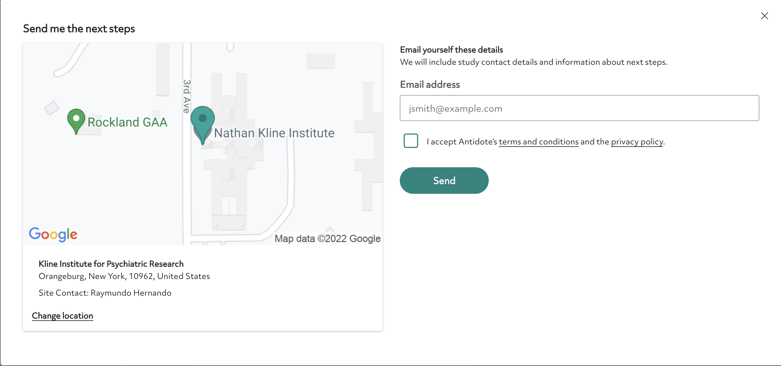
Once you contact the site, you will learn more about the study and find out if you may be a fit.
Don’t forget to enter your email address in the personalized trial alerts box. We’ll notify you with other trials that you may be eligible for.
Ready to start your trial search? Start below.
Topics: For Patients

